Software
Current activity: a collaboration with the CoenLab with the aim of understanding how patterns of gene activity in biological organs influence the developing shape. The BanghamLab is focussed on the conceptual underpinning: concepts captured in computational growth models, experimental data visualisation and analysis.
Notes on documenting our software
Notes for Lab members on how to contribute to this Wiki and where to put downloads.
Matlab tip: searching a large data structure for a particular field. Clear the command window. Evaluate the structure to list all the fields, then use the usual control-f search tool on the command window.
Computational biology
Quantitative understanding of growing shapes: GFtbox
We developed GFtbox to allow us to model the growth of complex shapes with the ultimate goal: to understand the relationships between genes, growth and form.
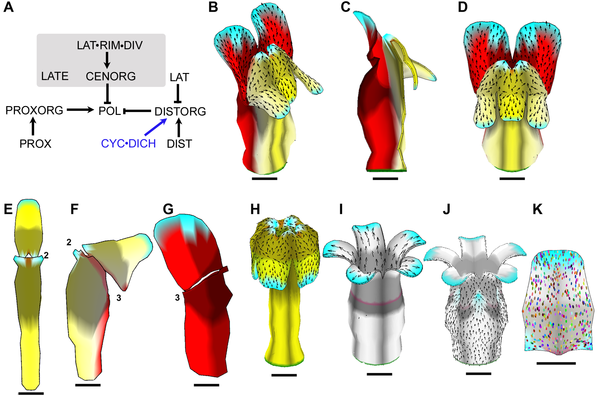 Example of a growing snapdragon flower and some mutants ( Green et al 2011). Growth is specified by factors (genes) according to the Growing Polarised Tissue Framework. Colours represent putative gene activity, arrows the polariser gradient and spots clones. Example of a growing snapdragon flower and some mutants ( Green et al 2011). Growth is specified by factors (genes) according to the Growing Polarised Tissue Framework. Colours represent putative gene activity, arrows the polariser gradient and spots clones.
|
GPT_Snapdragon_2010_Green_et_al-0002.png</wikiflv> |
| <imgicon>GPT_thumbnail2.png|120px|GFtbox</imgicon> |
For modelling the growth of shapes. Ready Reference Manual
What? How? Where? Background |
Viewing and measuring volume images: VolViewer
| <imgicon>VolViewer-logo.png|120px|VolViewer</imgicon> | For viewing and measuring volume images on both normal and stereo screens. Typical images from: confocal microscope and Optical Projection Tomography (OPT) images What? How? Where? Cell: Online Gallery | Front cover: Handbook of Plant Science | Front cover: The Plant Cell | American Scientist | Royal Microscopical Society: Infocus Magazine | Bundled with the Bioptonic 3001 scanner: Bioptonics Viewer | The Daily Mail | The Guardian newspaper: 3D Fruit fly | Qt Ambassador program | Triffid Nurseries website
|
VolViewer is used as a stand-alone app. or as a viewport for other systems, e.g. Matlab programs. VolViewer uses OpenGL and Qt to provide a user friendly application to interactively explore and quantify multi-dimensional biological images. It has been successfully used in our lab to explore and quantify confocal microscopy and optical projection tomography images. Written by Jerome Avondo it is open-source and is also compatible with the Open Microscopy Environment (OME) (Chris Allen and Avondo, et. al. OMERO: flexible, model-driven data management for experimental biology Nature Methods 9, 245–253 (2012)) ). ).
|
Analysing shapes in 2D and 3D: AAMToolbox
| <imgicon>AAMToolbox_logo.jpg|120px|AAMToolbox</imgicon> | For analysing populations of shapes and colours within the shapes using principal component analysis. What? How? Where?
|
The AAMToolbox enables the user analyse the shape and colour of collections of similar objects. Originally developed to analyse face shapes for lipreading (Matthews et al. 2002version of pdf), we have used it extensively for analysing the shapes of leaves (Langlade et al 2005.,Bensmihen et al. 2010) and petals (Whibley et al 2006,Feng et al. 2010). The analysis can be applied to art, for example, finding systematic differences between portraits by Rembrandt and Modigliani. |
Analysing the shapes of clones: SectorAnalysisToolbox
| <imgicon>Sector analysis icon.jpg|120px|SectorAnalysisToolbox</imgicon> | For analysing the shapes of marked cell clones. What? How? Where? |
The SectorAnalysisToolbox enables the user analyse the shapes of marked clones in a sheet of tissue. |
Algorithms
MSERs, extrema, connected-set filters and sieves
The algorithm finding MSER's starts with a connected-set opening or 'o' sieve
Comparison of Matlab MSER's and 'o' sieve Essentially, no difference.
Finding interest points, features and segmenting images.
- Technical briefing MSER's incorporate 'o' sieves.
- The twist: from restricted median filters to sieves and MSER's
- One dimensional sieves (measure length)
- Types of 1D sieve
- First applied to hydrophobicity plots but lets exploit their idempotency.
- Two dimensional sieves (measure areas)
- Properties
- Relation to MSER's
- Three dimensional sieves (measure volumes)
- Segment by volume instant gratification.
Art, extrema of light and shade: PhotoArtMaster
Art created using ArtMaster, and ArtMaster itself was featured in an exhibit at the London Victoria and Albert (V&A) Museum exhibition 'Cheating? How to make Perfect Work of Art' (2003). The exhibition centered on the idea of Hockney's that advances in realism and accuracy in the history of Western art since the Renaissance were primarily the result of optical aids such as the camera obscura, camera lucida, and curved mirrors. My exhibit used a touch screen (rare in those days) and ArtMaster to help visitors create 'paintings' from photographs. finding its name. (It is entirely different in principle from the software more recently used by Hockney to paint with an iPad.)
 
|
PhotoArtMaster
Saturday 07/06/2014: Inspired Photographer of the Year 2013 Tony Bennett when asked whether his photograph Mists and Reflections had been Photoshopped replied something like
"A digital camera delivers an unemotional raw image of pixels that you have to manipulate to create your photograph" Photographers manipulate as little as possible.
However there is another path one that creates pictures. For this you need another piece of software: PhotoArtMaster (ArtMaster). Professional Photographer said "Forget any comparison whatsoever with the art filters in Photoshop - this software reaches out and enters different stratospheres" Professional Photographer.
Early versions of PhotoArtMaster are still available from Amazon at low prices (I'm not sure where they come from.)
[1] . Some help for both the early versions and the latest version can be found in 'this document ).
Links to third party PhotoArtMastered pictures
- Oliver Bangham Colouful rounded shapes from, yes, my brother.
- The entry sequence of the comedy film 'Wimbledon'
The final version of the Windows ArtMaster2.0 is downloadable here with no support.
Unzip into (for example) the "Program Files" directory then set your system environment to include: C:\Program Files\Pam2.0 Release\jre\bin;C:\Program Files\Pam2.0 Release\bin; (You may need help for this. I right clicked 'computer' from the 'Start' menu, then selected 'Advanced system settings', then 'Environment Variables' and finally slid through the System variables until I found and selected 'Path'. This allowed me to edit the path by adding ';C:\Program Files\Pam2.0 Release\jre\bin;C:\Program Files\Pam2.0 Release\bin' to the end). Rather detailed help using the software is available in 'this essay .
The sieve algorithms underpinning PhotoArtMaster software are described in an extract of the
'essay . These documents were written to support our Fo2Pix company. PhotoArtMaster originally sold >65,000 licences but ill health forced the closure of Fo2Pix.
|
Reaction-diffusion and morphogenesis
Illustration of morphogenesis inspired by Turing's paper.
Example using growth toolbox GPT_ReactionDiffusionTentacles_20121211.zip
1 A
Open source systems to which we have contributed
OMERO
| <imgicon>OMERO_DIAGRAM.jpg|100px|OMERO</imgicon> | For working with the OME image database. See Details, Download OMERO Workshop (Windows, Mac, Linux) |
Open Microscopy Environment Remote Objects (OMERO). for visualising, managing, and annotating scientific image data. See also our OMERO Workshop training course we ran in April 2011. |
Tools and Utilities
BioformatsConverter
| <imgicon>BioformatsConverterZip.png|100px|BioformatsConverter</imgicon> | For converting microscope manufacturer proprietary file formats. See Details (Windows, Mac, Linux) |
This tool allows for the batch conversion of microscope manufacturer proprietary file formats, to the open source OME-TIFF standard. Uses the Bioformats library. |
Dependency Checking Tool
Tool for recursively finding what further functions a function depends on. See Details
PIN Point
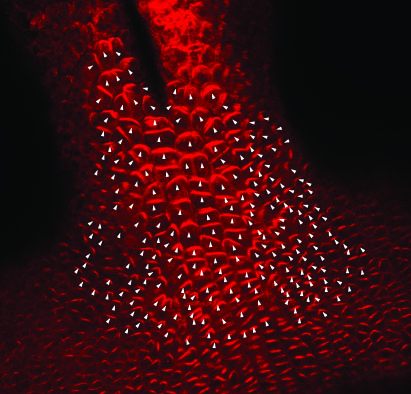
|
Tool for analysing PIN Signal in cells. | Download Sample Project |
In development
MTtbox
| <imgicon>MTtboxA.jpg|100px|BioformatsConverter</imgicon> | For modelling the behaviour of microtubules within a cell. See Details |
In development. The idea is to be able to model the behaviour of growing microtubules and factors as they react chemically and diffuse within the different cell compartments. The icon shows a spherical cell sliced open to show concentric components: cell wall (magenta), plasma-membrane (yellow), cytoplasm (green) and vacuole (yellow). Microtubules (blue) grow in 3D within the cytoplasm. |
Historical
Robot arm: still in production after 30 years (serving local industry)

|
For teaching production control and interrupt programming. 1983 and we are in a world of Apple II and BBC B computers - the 6502 processor reigns. Particularly good for real-time control it responded very fast to hardware interrupts from, for example, the timer. To illustrate timer interrupts what better than digital servo-motors? Set up the on-board timers to produce a stream of 'heartbeat' of pulses, one every 20 ms out of the parallel port and control up to eight motors. Pulse widths, from 1 to 2 ms, control the position of each motor arm. Derek Fulton and I made some loose lab. money by writing a series of articles showing exactly how to build and, in particular, control this robot arm.Bangham et al.. Our copyright, I took it to a local company LJ-Electronics (now LJ-Creative) who incorporated it detail for detail into their product line. Originally, they called it the Emu. Still in production: Lovely outcome. |